Vaccine and pharmaceutical formulation resources
Advancing vaccine and pharmaceutical formulation research and technologies to improve the efficacy, stability, safety, and ease of use of essential medicines and vaccines that prevent and treat diseases.
About PATH's work in formulation
PATH collaborates with researchers and vaccine manufacturers to accelerate the development and availability of new and lifesaving vaccines that meet international standards for quality, safety, and efficacy and are affordable for low- and middle-income countries (LMIC).
With more than 40 years of experience, we provide gap-filling technical support for high-potential innovations at each stage of product development, from early-stage discovery to preclinical screening, manufacturability demonstration, and process technology transfer. We adapt, test, and validate emerging concepts, technologies, and methods that optimize vaccine efficacy, stability, safety, and ease of use.
Connect with us via email at: innovation@path.org
Technology Updates
Formulation innovations
mRNA vaccine development
-mRNA potency assay development
-Comparative evaluation of mRNA-lipid nanoparticle (LNP) manufacturing platforms
-Thermostable formulation platforms for mRNA-LNP based vaccines
-Sensitive immunoassay to support development of COVID-19 vaccines
Thermostable formulations
-Buccal naloxone for treatment of opioid overdose
-Thermostable oral insulin for diabetes management
PATH has strong expertise in developing thermostable formulations for different types of vaccines and drugs to protect these valuable products from temperature extremes.
PATH generates sensitive qualitative and quantitative assays in accordance with International Council for Harmonisation guidelines for measuring the contents of vaccines and drugs—critical for formulation development, stability monitoring, production, and final product release testing.
PATH is working to improve vaccine efficacy in existing and novel adjuvanted formulations for several vaccine candidates. Support activities include the development of assays for measuring the concentration and activity of adjuvants in vaccines and qualified procedures for production of adjuvants.
We provide broad mRNA platform support at varying stages of product development, with the aim of advancing mRNA technology for high-priority vaccines and therapeutics and of building expertise and local manufacturing capacity in low- and middle-income countries. We develop customized lipid nanoparticle (LNP) formulations and conduct analytical and stability testing to support preclinical immunogenicity and efficacy studies.
PATH conducts in-house biotechnology work on vaccine and pharmaceutical technologies, including mRNA in our on-site, custom-built biosafety level 2 (BSL-2) isolation laboratory. Click through the galley to see mRNA vaccine development equipment.

mRNA equipment: the Precigenome Flex-S, a microfluidic chip-based platform that produces mRNA-LNPs at a small production scale to generate mRNA-LNPs as this platform is quick, easy to use, repeatable, reliable, and scalable.
1 of 8
The Precigenome Flex-S is operated via touch screen, requiring only a short number of steps to produce a batch of lipid nanoparticles.
2 of 8
Formulation laboratory - mRNA vaccine development equipment high-performance liquid chromatography (HPLC) unit the Thermo Scientific Ultimate 3000. PATH uses HPLC for RNA identity, purity, lipid, and impurity analysis.
3 of 8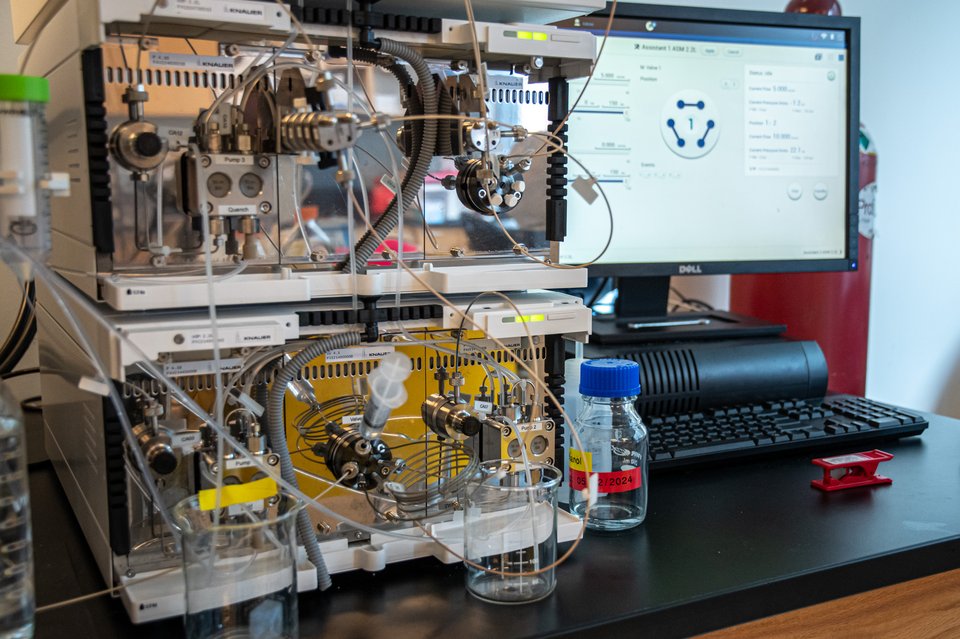
Knauer Impingement Jet Mixer for lipid nanoparticle (LNP) production. Knauer is an impingement jet mixing platform that encapsulates mRNA in LNPs by colliding the two materials at a high velocity, yielding nanoparticles that match key quality attributes.
4 of 8
Formulation laboratory - mRNA vaccine development equipment: Knauer Impingement Jet Mixer for lipid nanoparticle production.
5 of 8
Formulation laboratory - mRNA vaccine development equipment: Knauer Impingement Jet Mixer for lipid nanoparticle production. The operators pictured are Changcheng Zhu, at the time a senior program officer, together with Estelle Neathery.
6 of 8
Formulation laboratory - mRNA vaccine development equipment: Knauer Impingement Jet Mixer for lipid nanoparticle (LNP) production. The Knauer platform is used to fulfill grantee requests that require a large volume of LNP.
7 of 8
Formulation laboratory - mRNA vaccine development equipment: Knauer Impingement Jet Mixer for lipid nanoparticle production.
8 of 8Collaborating for impact
Global partnerships with manufacturers and academic institutions
PATH’s formulation team offers technical advice and services to collaborators, including product development partners, on formulation development, assay development, and reagent production for supporting vaccine and pharmaceutical candidates in development. We engage with partners in early development and provide continued support through phase II trials, including formulation and assay support for clinical studies and transfer of technology to manufacturers.
Our formulation team has conducted more than a dozen successful technology transfers involving formulation, assay, and adjuvant production to multiple manufacturers on five continents.
Our capabilities in production of and analytical methods for mRNA-LNPs
In-house capabilities: Lab-scale LNP manufacturing platforms. Analytical testing.
Custom mRNA-LNP production: Fulfillment of end-to-end custom requests for non-GMP mRNA-LNP material to enable BMGF grantees to advance their mRNA vaccine candidates through preclinical studies.
Contract manufacturing and testing labs: Cryo-TEM. In vivo bioluminescent imaging. Preclinical immunogenicity studies.
Overview of mRNA vaccine development
PATH collaborates with researchers and vaccine manufacturers to accelerate the development and availability of new and lifesaving vaccines that meet international standards for quality, safety, and efficacy and are affordable for low- and middle-income countries.
We adapt, test, and validate emerging concepts, technologies, and methods that optimize vaccine efficacy, stability, safety, and ease of use.
Our mRNA areas of focus:
mRNA characterization
mRNA drug substance
mRNA drug product
mRNA stabilization
Artificial intelligence in mRNA vaccine development
Take the survey: Endotoxin testing methods
Endotoxin testing is essential for ensuring the safety of injectable pharmaceutical products by detecting potentially harmful bacterial toxins.
This survey aims to review current endotoxin testing methods and assess the potential for the replication Factor C (rFC) method to be adopted by manufacturers in LMICs. By evaluating the benefits and challenges of rFC technology, this survey seeks to provide valuable insights into how LMIC manufacturers, regulators, and stakeholders might navigate the transition to this innovative method, enhancing their testing capabilities while addressing global sustainability and ethical considerations
Questionnaire for manufacturers Take the survey here.
Questionnaire for regulators. Take the survey here.
Research
Recent research: journal articles
Safety and immunogenicity of an egg-based inactivated Newcastle disease virus vaccine expressing SARS-CoV-2 spike: Interim results of a randomized, placebo-controlled, phase 1/2 trial in Vietnam. Vaccine Volume 40, Issue 26.
Preclinical Safety and Pharmacokinetics of Heat Stable Oxytocin in Sublingual Fast-Dissolving Tablet Formulation.Pharmaceutics Volume 14, Issue 5.
Contact us
Connect with us if you are interested in collaborating on vaccine and pharmaceutical formulation innovations.
Leaders
-
![]()
Courtney Jarrahian
Global Program Leader
-
![]()
Dr. Manjari Lal
Portfolio Leader: Formulation Technologies
-
![]()
Dr. Jessica White
Evaluation and Research Laboratory Lead






