Semi-synthetic artemisinin for malaria treatment
Diversifying the artemisinin supply to protect and increase access to lifesaving antimalarial therapies.
In most malaria-endemic countries, the primary treatment for malaria is artemisinin-based combination therapy (ACT)—reliable access to which is critical for malaria patients. To keep this lifesaving treatment accessible, a stable and resilient supply chain is vital. However, the vegetal supply of artemisinin, the key starting material for these treatments, is vulnerable to several risks, including:
- Price volatility, common in commodity markets like oil, for example, that could hurt the ability of manufacturers to keep the supply of ACTs stable, affordable, and available to patients globally.
- Weather- and climate-related risks that can impact artemisia annua crops, the plant that vegetal artemisinin is derived from, which threatens the ability of manufacturers to meet demand affordably and reliably.
- Production concentrated largely in one country, which yields 97% of all artemisinin used in global health supply chains.
Since 2022, PATH has led an effort to understand the current state of the market for vegetal and semi-synthetic artemisinin (SSA) within the antimalarial commodity market and advocate for a diversified artemisinin supply made up of a mix of SSA and vegetal approaches.
The opportunity to build a resilient antimalarial supply chain
SSA is a potential solution to the market and supply chain challenges associated with vegetal artemisinin because:
- It can help mitigate the risks posed by the geographic concentration of vegetal artemisinin production as it is not dependent on crop yields, contributing to a more secure supply chain.
- It is not vulnerable to weather- and climate-induced disruptions, enabling stable sourcing even as extreme weather events and other challenges of a changing climate persist.
- It could create opportunities to support the sustainable local production of this essential medicine in communities that need it most, by transferring technology to regional manufacturers.
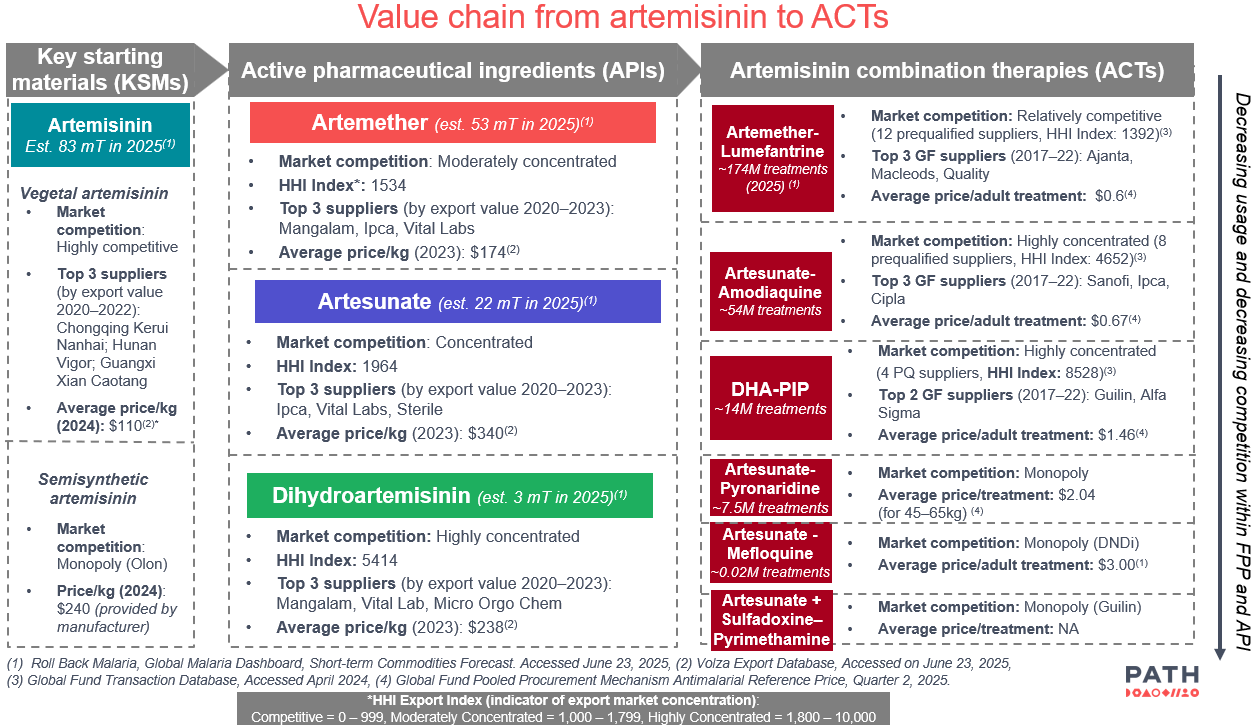
Value chain analysis conducted by PATH's Market Dynamics Program as part of ongoing efforts to understand the challenges associated with SSA uptake.
Overcoming the barriers hindering the adoption of semi-synthetic artemisinin
Ensuring a diversified artemisinin supply made up of a mix of SSA and vegetal artemisinin is critical in anticipation of an unpredictable crisis for the reasons outlined above. Additionally, it is important to note that SSA cannot be immediately procured at a moment’s notice due to relatively long production timelines. Therefore, dependable demand is necessary to ensure production lines remain active and SSA is available when needed. This is like fire insurance, you need to have it before your house is on fire.
However, manufacturers of ACTs have been slow to adopt SSA for use in their products. Even though vegetal artemisinin prices have been historically volatile, PATH has received consistent feedback in conversations with manufacturers that the current incentives to use SSA cannot sufficiently overcome the current price gap between SSA and vegetal artemisinin, preventing the diversification of their artemisinin supply.
To overcome these barriers, PATH advocates for increased collaboration, commitment, and sharing of responsibilities between international agencies, manufacturers, and global procurers, as well as growth in the number of manufacturers that develop SSA to create more competition and lower prices. PATH has also developed market analyses of the artemisinin and ACT market to increase understanding of challenges faced in these markets and the role SSA can play to diversity supply chains.
Safe and affordable ACTs save the lives of millions of malaria patients—many of whom are children under the age of five—every year. Increasing the uptake of SSA has great potential to keep these lifesaving therapies accessible.
PATH's history with SSA
PATH's involvement with SSA goes back to 2004 when the organization spearheaded the first cross-sector partnership to stabilize the antimalarial drug supply and prevent future shortages.
With funding from the Gates Foundation, PATH and its partners set out to develop a new pharmaceutical manufacturing process to produce commercial volumes of high-quality, non-seasonal, and affordable artemisinin to supplement the plant-based supply.
During the project’s final phase, PATH and Sanofi, the project’s development and manufacturing partner, successfully moved SSA from research lab to the factory floor, and on to the global market.
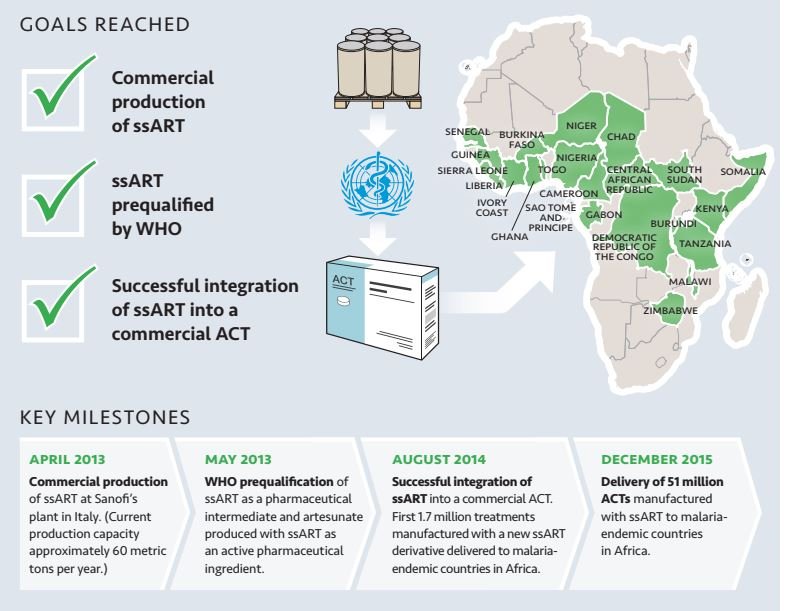
Key milestones included:
- April 2013 - commercila production of SSA at Sanofi's plant in Italy
- May 2013 - WHO prequalification of SSA as a pharmaceutical intermediate and artesunate produced with SSA as an active pharmaceutical ingredient
- August 2014 - successful integration of SSA into a commercial ACT and first 1.7 million treatments manufactured with a new SSA derivateive delivered to malaria-endemic countries in Africa
- December 2015 - delivery of 51 million ACTs manufactured with SSA to malaria-endemic countries in Africa
About the partnership: The partnership for semisynthetic artemisinin was led by PATH’s Drug Development program. The project began in 2004, and partners included Amyris, Sanofi, and the University of California, Berkeley (UC Berkeley). The novel use of synthetic biology technology was based on pioneering inventions from UC Berkeley, Amyris, the National Research Council Canada Plant Biotechnology Institute, and GenoClipp Biotechnology BV. The chemistry expertise and the industrial experience and capacity of Sanofi helped bring this project from small laboratory experiments to production on the factory floor.