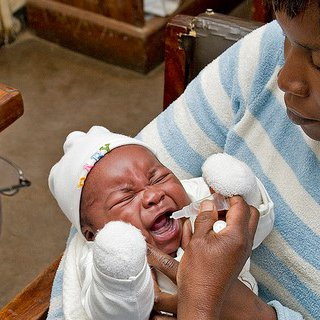
Rotavirus remains a leading cause of severe diarrhea among children younger than five years old, particularly in low- and middle-income countries (LMICs). While several globally available, live attenuated, oral rotavirus vaccines (LORVs) have significantly reduced illness and deaths due to rotavirus, their efficacy in LMICs has been suboptimal and additional tools are needed to close this gap.
This inspired the Gates Foundation to invest in the development of injectable rotavirus vaccine candidates aimed at enhancing protection in high-burden rotavirus settings. In 2011, PATH began receiving support from the foundation for a non-replicating rotavirus vaccine (NRRV) project to develop a new injectable rotavirus vaccine for infants in LMICs.
PATH’s NRRV project, which is now drawing to a close, featured collaborations with many global health researchers and institutions with the aim of delivering an injectable rotavirus vaccine with superior efficacy compared to existing LORVs. This approach represented a bold departure from traditional LORVs and required a fundamental rethink of rotavirus protection.
“Innovation is at the heart of PATH’s work, so the NRRV project was a natural fit for our team,” said Dr. John Konz, Global Head of Viral Diseases in PATH’s Center for Vaccine Innovation and Access. “We took on the challenge of improving rotavirus vaccines with the ultimate goal of providing better protection to those most vulnerable to severe illness and death due to this disease.”
Innovation in design, execution, and scale
Scientists don’t know for sure why LORVs have decreased efficacy in high-burden rotavirus settings—which are mainly in LMICs—but it's most likely multifactorial, involving the oral route of immunization and gut-related issues. An injectable vaccine could potentially circumvent these factors and provide increased protection.
PATH and partners have been working for nearly 15 years to develop an injectable NRRV, beginning with preclinical research on a highly promising vaccine candidate, then moving to safety and immunogenicity testing, and finally a multi-country efficacy study. Initially a monovalent (one strain) candidate, the formulation was expanded to include three rotavirus strains to broaden protection against the most prevalent strains in LMICs.
The clinical development journey was ambitious and accelerated. A Phase 1 study in the United States and a Phase 2 study in South Africa demonstrated that the monovalent NRRV candidate was safe and immunogenic. Subsequently, a Phase 2 trial conducted in South African adults, toddlers, and infants confirmed safety and robust immunogenicity of the trivalent vaccine candidate, called trivalent P2-VP8.
These results paved the way for a pivotal Phase 3 efficacy study—a clinical trial conducted in Ghana, Malawi, and Zambia that enrolled more than 3,000 healthy infants. The trial was designed to evaluate trivalent P2-VP8’s safety, immune responses, and efficacy relative to a licensed oral rotavirus vaccine, ROTARIX®.
Final results from the study, which was the first to evaluate the efficacy of an injectable rotavirus vaccine candidate, were recently published in the peer-reviewed journal Vaccine. The results show that the vaccine candidate was safe and well tolerated, with no safety concerns indicated. However, efficacy data revealed that trivalent P2-VP8 was inferior to ROTARIX in preventing severe rotavirus gastroenteritis (SRVGE). The relative vaccine efficacy of trivalent P2-VP8 against SRVGE was -77.68% and against rotavirus of any severity was -47.78%.
“While this is not the outcome we hoped for, these data provide important evidence for the scientific community,” said Dr. Stanley Cryz, Director of PATH’s NRRV project. “This does not rule out the possibility of an effective injectable NRRV being developed in the future. We hope these results will continue to push the field forward toward the goal of a highly effective injectable rotavirus vaccine for high-burden settings.”
“We hope these results will continue to push the field forward toward the goal of a highly effective injectable rotavirus vaccine for high-burden settings.”— Dr. Stanley Cryz, Director of PATH’s NRRV project
In parallel with the Phase 3 trial, PATH also conducted a Phase 2 study in South Africa to see if different vaccine regimens combining the administration of oral rotavirus vaccines and injectable trivalent P2-VP8 could improve immune responses. Results from this study will be available later this year.
Results from these trials continue to fuel further investigations to improve rotavirus vaccines. PATH is conducting laboratory-based analyses, with various partners, using samples collected during the Phase 3 trial to better understand what type of immune response is associated with protection from rotavirus disease to aid future vaccine development efforts.
Lessons to inform future research
While the trivalent P2-VP8 vaccine candidate did not meet its efficacy goals, the project’s legacy will have lasting implications. Among a number of important key takeaways, the project demonstrated the safety and tolerability of injectable rotavirus vaccine candidates in infants.
“These important insights will certainly inform next-generation rotavirus vaccine strategies,” said Dr. Carl Kirkwood, a former Senior Program Officer at the Gates Foundation who oversaw PATH’s NRRV project. “Researchers around the globe are continuing to explore alternative delivery platforms, adjuvants, and antigen combinations to enhance rotavirus vaccine efficacy. While the results were disappointing, PATH’s findings provide a basis upon which future injectable rotavirus vaccine development can be built.”
Dr. Kirkwood’s leadership was instrumental in the success of PATH’s NRRV project over the last decade, and we’re grateful for his steadfast support. His expertise in the field of rotavirus vaccines provided PATH with critical guidance through a challenging and complex vaccine development pathway for this innovative rotavirus vaccine approach.
A legacy of bold science
Given the discouraging efficacy results, PATH’s NRRV project is coming to a close. The project may not have delivered a new licensed vaccine, but it was a successful trailblazer in rotavirus immunization science. Expert conduct of the study site teams at each of the clinics, along with the Gates Foundation’s funding support and thought partnership, were essential to the project’s success in driving forward this pioneering body of research.
“As the most advanced injectable rotavirus vaccine candidate, our work on the trivalent P2-VP8 candidate laid the groundwork for future breakthroughs and underscored the importance of innovation, even when outcomes are uncertain,” said Dr. Cryz. “As global health leaders and scientists continue to seek better tools for protecting children from rotavirus, we hope that the lessons from this project will help guide the way for years to come.”