Partnership for Acceleration of Innovative Diagnostics for Malaria (PARADIGM)
Early and accurate diagnosis of malaria is essential for case management and surveillance. In collaboration with national malaria programs, manufacturers, and global stakeholders, the PATH Partnership for Acceleration of Innovative Diagnostics for Malaria (PARADIGM) project is advancing the availability of sensitive diagnostic tests that address current and evolving needs to diagnose, control, and eliminate malaria.
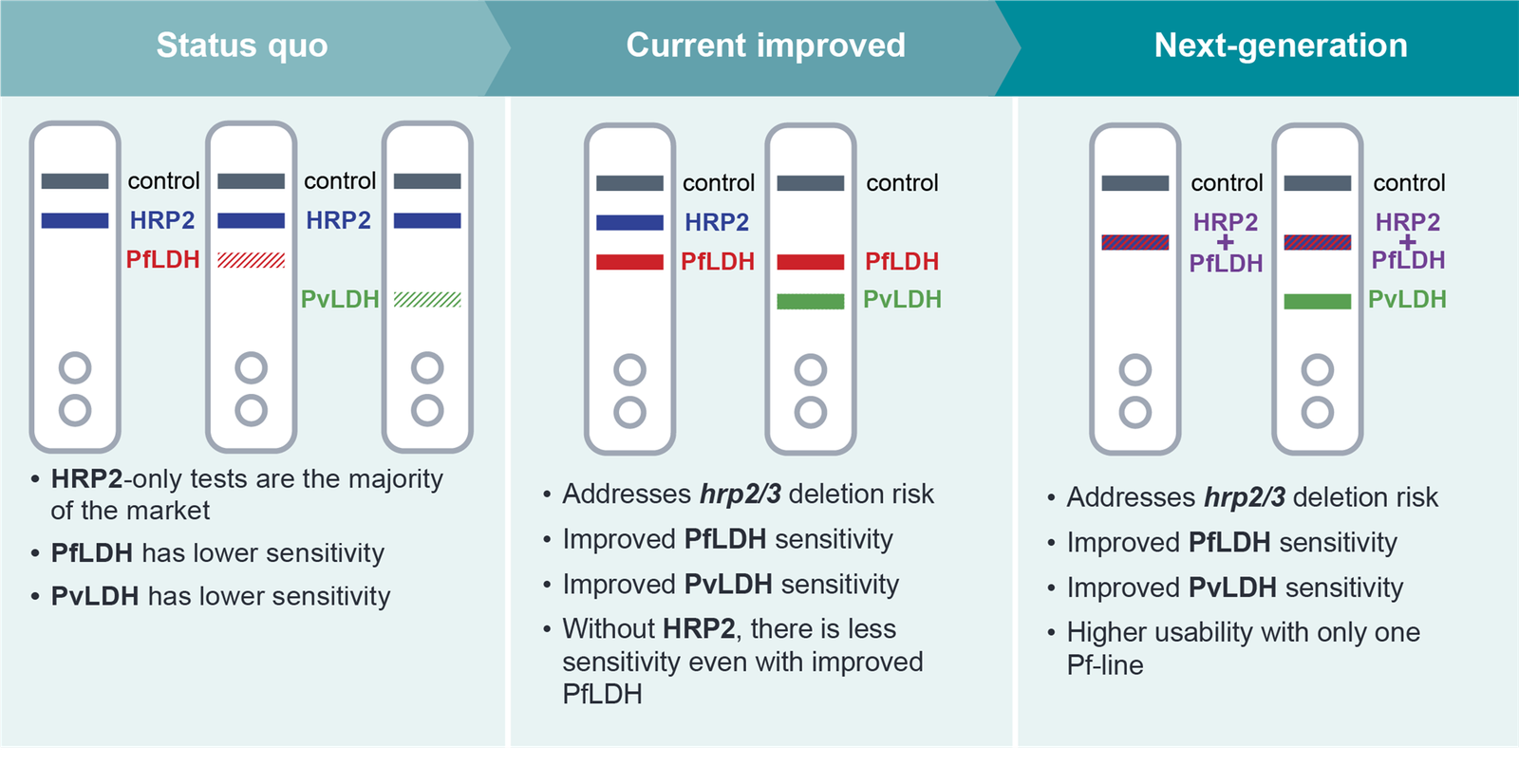
Schematic of status quo RDTs to next generation RDTs.
About the project
PARADIGM supports manufacturers’ efforts to develop more reliable RDTs that will enable national programs to reach their malaria control and elimination goals. Our work supports product development throughout the product development cycle including identification and assessment of novel biomarkers, research and development (R&D), clinical validation, and market introduction. See PARADIGM project 2-pager for more information.

Lab-to-market pathway
Resources
Clinical Research Tools
- Clinical study protocol template, data collection tools, and other associated resources to support malaria RDT clinical evaluations
- Repository of data from clinical studies, including malaria antigen levels in clinical samples
- Protocols for use of WHO International Standards as quality controls for malaria RDTs
- Microscopy resources
- Antigen quantification assays
- Protocols for use of WHO International Standards for the harmonization of malaria nucleic acid amplification techniques (NAAT)



Read more of our published work here.
Contact
Contact dxinfo@path.org for more information.
Acknowledgement
This project is supported by the Gates Foundation. Their support enables us to strengthen diagnostic innovations and access to improve health outcomes.