An improved vaccine to help end polio
A next-generation oral polio vaccine, developed by PATH and partners, is bringing the world closer to ending polio—only the second human disease in history that could be wiped out completely.
The original oral polio vaccine was a major success in reducing global polio cases. However, in rare situations—especially in areas where few people have been vaccinated—the weakened virus in the vaccine could mutate and cause outbreaks. To tackle this, PATH scientists and partners advanced the novel oral polio vaccine type 2 (nOPV2), a more genetically stable version of the vaccine. It’s designed to protect with reduced risk of reverting into a harmful form.

A nurse vaccinates a child against polio. Photo: Gates Foundation/Riccardo Gangale.
1 of 3 2 of 3
2 of 3
 3 of 3
3 of 3
Since 2020, over 1 billion doses of the new vaccine have been given to children at risk from poliovirus outbreaks. PATH led the consortium that developed nOPV2, coordinating partners and efforts across the globe. This leadership ensured fast and effective progress, from early research to real-world impact.
In November 2024, PATH’s Dr. John Konz accepted the Innovating for Impact Partnership Award on behalf of the project. The award, presented by the Global Health Technologies Coalition, honors scientific innovations that address urgent global health needs. “It has been an honor to help move this vaccine from a lab idea to a global health solution,” said Dr. Konz. “We do this work to save lives and create a healthier future.”
Delivering hope to islolated children in the DRC
Delivering vaccines is no small feat in Haut Lomami, a remote province in the Democratic Republic of the Congo (DRC). The province’s isolated islands and riverbank villages are hard to reach, except by canoe or rugged paths. As a result, many children here have never received even a single vaccine.
To change that, PATH supported the DRC government and its Expanded Programme on Immunization to launch the Congo River Strategy—a bold campaign to reach isolated children with lifesaving vaccines.
The effort began with mapping hard-to-reach communities and deploying 54 special vaccination teams by land and river.

Héritier, a vaccination team leader, administers a dose of polio vaccine to a young child. Photo: PATH/Yves Ndjadi.
1 of 4 2 of 4
2 of 4

Teams travel by boat to reach remote island communities and vaccinate children against polio and other preventable diseases. Photo: PATH/Yves Ndjadi.
3 of 4 4 of 4
4 of 4
One of those teams is led by Héritier, a community health worker and vaccination team leader who travels hours by canoe to reach distant islands, where children have little or no access to health care. “Every time we go on a mission, we know we’re not just vaccinating. We are bringing hope,” says Héritier.
On one island, a mother named Marie waited two days for the team’s arrival. “Vaccination is the only way to protect our children,” she said, as her four children received the polio vaccine.
Over 11 months, the campaign vaccinated more than 30,000 children with over 100,000 doses of routine vaccines. By supporting the DRC’s vaccination goals, PATH is helping children have brighter futures.
A tiny patch that protects kids from measles and rubella
Measles is one of the most contagious diseases in the world. Rubella, while less known, can cause serious birth defects. But both are entirely preventable with vaccines.
To stop measles from spreading, at least 95 percent of people need to be vaccinated. Yet globally, only about 85 percent of people have been vaccinated. One challenge is that current measles-rubella vaccines must be kept cold—making it hard to reach remote communities.
PATH and partners are working on a new solution: microarray patches (MAPs). These tiny patches deliver the vaccine through small projections that dissolve into the skin—no needles required. And, because MAPs are more heat-stable than traditional vaccines, they may not need constant refrigeration. That could make it easier to bring vaccines to remote areas and hard-to-reach communities.
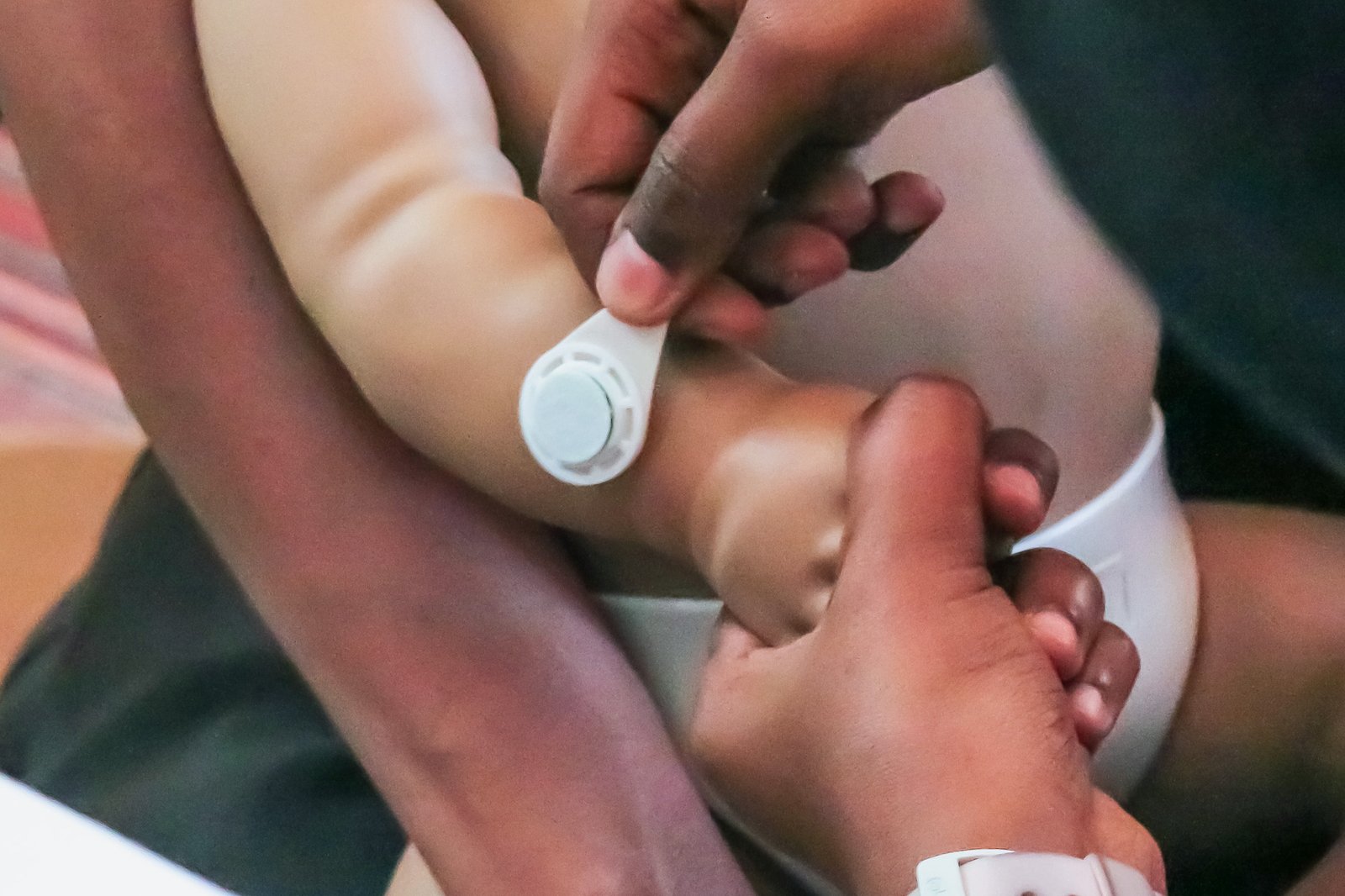
A user study participant applies a microarray patch to a manikin as part of a simulated use assessment. Photo: PATH.
In fact, MAPs could help prevent 35 percent of measles cases and reach up to 80 million additional children between 2030 and 2040.
In 2024, PATH gathered input on two MAP options in the DRC, Kenya, and Nepal. Feedback from health workers and caregivers was overwhelmingly positive—they found the patches easy to use, less painful, and safer than traditional shots.
Their input is now shaping the design of further research, bringing this promising tool closer to the children who need it most.
A new test transforms the fight against malaria
A new blood test is transforming the fight against one of malaria’s most persistent forms: Plasmodium vivax. Unlike other types, P. vivax can hide in the liver and trigger relapses weeks or months after treatment. To fully cure it, patients need a special medication that targets these dormant parasites.
But there’s a catch. This medication can cause serious harm to people with a genetic condition called G6PD deficiency, which affects over 400 million people worldwide. Without a way to test for G6PD deficiency, many patients either miss the full treatment or face dangerous side effects.
That’s changing with the STANDARD G6PD Test, developed by SD BIOSENSOR and PATH. It’s the first point-of-care G6PD test to be prequalified by the World Health Organization (WHO), meeting global standards for safety and accuracy.

Health workers in Laos test the usability of a prototype G6PD point-of-care test. Photo: PATH/Spike Nowak.
1 of 3 2 of 3
2 of 3

The STANDARD G6PD Analyzer, a point-of-care test that protects patients and aids malaria elimination. Photo: PATH.
3 of 3The test uses a simple finger-prick blood sample to deliver results in just two minutes. It’s, easy to use and designed for remote and low-resource settings where most malaria cases occur and traditional lab-based testing isn’t feasible.
WHO prequalification paves the way for more countries to adopt the test widely and integrate it into national malaria programs. With this innovation, health workers can safely provide full treatment for P. vivax to women, men, and children, helping prevent relapses and moving us closer to a malaria-free future.
Simple devices, big impact: Improving child health outcomes
In northeastern Tanzania, clinical officer Joyce Nuhu remembers a mother named Aisha who brought her child to Nguvumali Health Center in distress. The child was struggling to breathe and wouldn’t eat. The diagnosis: pneumonia. Thanks to Aisha’s quick action and timely care, the child recovered. But in many places, children facing similar symptoms aren’t as fortunate.
Across low- and middle-income countries, severely ill children often go undiagnosed or mistreated due to a lack of basic tools like pulse oximeters, which detect dangerously low blood oxygen levels. Without them, health workers may miss signs of severe illness.

Fatina Msangi, a doctor at Duga Health Center in Tanzania, attends to a sick child using a pulse oximeter. Photo: PATH/Olgah Odek.
1 of 3 2 of 3
2 of 3
 3 of 3
3 of 3
PATH’s Tools for Integrated Management of Childhood Illness (TIMCI) project addressed this gap between 2019 and 2024 in India (Uttar Pradesh), Kenya, Senegal, and Tanzania. The project introduced pulse oximeters and digital decision-support tools in frontline clinics, making it easier for health workers to diagnose and treat childhood illnesses accurately.
Across the four countries, more than 1,400 providers were trained and over 200,000 children received improved care. Joyce says these tools now help her assess children faster and more confidently.
The TIMCI project proves that simple, cost-effective technologies—combined with training—can save lives. It offers a roadmap for expanding access to quality child health care.
One dose, many lives: Expanding access to HPV vaccines
Cervical cancer kills more than 350,000 women each year—mostly in low- and middle-income countries. The primary cause is infection with the human papillomavirus (HPV), but the disease is preventable with vaccination.
Unfortunately, many countries face barriers to HPV vaccination, including high costs, limited supply, and logistically challenging multi-dose schedules. PATH is working to change that.
Partnering with the vaccine manufacturer Innovax, PATH helped evaluate a more accessible option: the Cecolin® bivalent HPV vaccine (bivalent vaccines stimulate an immune response against two virus variants). PATH supported Innovax through Cecolin’s WHO prequalification and initiated clinical trials in Bangladesh and Ghana.

School girls in Bangladesh line up to be vaccinated against HPV during a PATH-supported vaccine introduction campaign. Photo: PATH/Maksudur Rahman.
The results, shared with WHO in 2024 and published in 2025, were game-changing. The study showed that just one dose of Cecolin can trigger a strong immune response. This evidence informed WHO’s recommendation of a single-dose schedule for Cecolin, making it easier and more affordable to protect more girls.
By making HPV vaccines more affordable and easier to deliver, PATH is helping prevent cervical cancer and protect millions of girls—one dose at a time.
Cecolin is a registered trademark of Xiamen Innovax Biotech Co., Ltd.
From comprehensive primary health care to support services for infectious and chronic diseases, PATH makes good health more accessible by improving the care people receive.
From training health workers to advancing effective policies, PATH partners with governments and local organizations to strengthen health systems at every level.
2024 PATH annual report
Annual report home | Product development and access | Health and disease management | Health systems strengthening | Financial summary | Leadership | Supporters
