The rollout of malaria vaccines across Africa, along with the use of insecticide-treated bed nets, medicines, and other interventions, is expected to help reduce malaria disease burden in the coming years. Nevertheless, scientists continue to research additional prevention tools to find even better options for defeating malaria.
Next-generation malaria vaccines have the potential to provide greater impact in preventing illness and death over the currently available vaccines for those most at-risk. Innovative biologics, such as monoclonal antibodies, may also offer short-term, high-level protection against infection and onward transmission of malaria, potentially in just a single injection. A recently published manuscript, commissioned by the World Health Organization (WHO) and led by PATH, provides an in-depth look at the potential public health, economic, and societal value that next-generation malaria vaccines and monoclonal antibodies can bring.
PATH’s current research and development efforts on malaria vaccines and biologics covers a range of approaches that focus on different stages of the Plasmodium parasite’s complex lifecycle to tackle it from multiple angles. Adding these new tools to the arsenal will be essential to making malaria eradication a reality.
Anti-infection malaria vaccines
The two current malaria vaccines, RTS,S/AS01 and R21/Matrix-M, protect against clinical malaria in young African children by preventing initial infection. They both target the same region of the circumsporozoite protein (CSP), which is the most abundant protein on the sporozoite, the parasite form that establishes the initial infection following a bite from an infectious mosquito. The sporozoites then migrate to the liver where they multiply, leading to the infection of red blood cells and symptoms of clinical malaria.
This is why RTS,S and R21 are called “anti-infection vaccines.” These types of vaccines elicit an immune response that either prevents the sporozoite from entering the liver and/or attacks the infected liver cell if infection does occur.
Leveraging our decades of work on malaria vaccine development, PATH is working with partners on exploring different vaccine regimens and dosages of these vaccines to improve their impact. We’ve also helped conduct research on the benefits of combining use of the vaccines with preventive malaria drugs (chemoprevention).
For example, a five-year study examined the benefits of combining RTS,S with antimalarial drugs in settings of highly seasonal malaria transmission. The results confirmed a two-thirds greater reduction in severe malaria cases and deaths for the vaccine-drug combination compared with either intervention alone. Other research aims to improve our understanding of the mechanisms by which RTS,S confers protection to vaccinated individuals to inform the development of more efficacious anti-infection vaccines for all age groups.
To further support this work, PATH is developing tools to help identify new anti-infection vaccine candidates. For example, we’re collaborating with partners on identifying CSP-specific biomarkers associated with protective immunity to serve as surrogate endpoints for vaccine licensure. We also worked with partners to establish a new preclinical model to allow for a head-to-head comparison of novel CSP-based vaccine candidates with current vaccines to better predict superior efficacy prior to entering costly clinical testing and support decision-making for future investment.
Novel vaccine candidates
Guided by the WHO’s preferred product characteristics for malaria vaccines, PATH is partnering on next-generation malaria vaccine candidates that aim to offer increased efficacy, improved durability of protection, lower cost, and fewer required doses compared to the currently available vaccines.
Blood-stage vaccine candidates target the malaria parasite at its most devastating stage—the rapid replication of the organism in human red blood cells. Blood-stage parasites cause clinical disease and all malaria-associated illness and death. Blood-stage vaccines aim to decrease the number of parasites in the blood (merozoites), and in so doing, reduce the severity of disease.
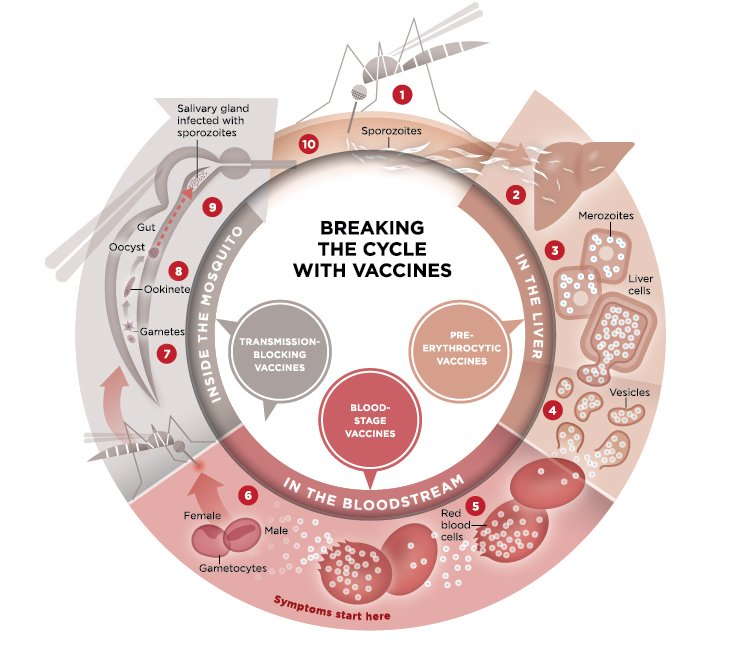
This illustration explains the malaria parasite life cycle and indicates the points where malaria vaccines can target different steps in the life cycle.
Evidence suggests people who have survived regular exposure to Plasmodium can develop natural blood-stage immunity over time. If successful, this class of vaccines could help minimize or even prevent the manifestations of Plasmodium clinical disease and may also help the body to develop natural immunity.
This promising area of research includes blood-stage vaccine candidates based on the RH5 protein, which can prevent the malaria parasite from entering human red blood cells. PATH supported preclinical research that led to the rational design of new constructs, as well as early-stage clinical research on RH5-based candidates.
Another next-generation approach is transmission-blocking vaccines (TBVs). TBVs would disrupt sexual reproduction of the malaria parasite within the mosquito, thereby preventing its onward transmission to the next human host when they take their next blood meal. While a standalone TBV would not provide a direct and immediate benefit to the vaccinated person, it would provide a delayed benefit via a community-wide reduction in the number of parasite-harboring mosquitoes.
PATH partnered on TBV work focused on defining critical regions of protective immunity on the two leading TBV candidates, Pfs230 and Pfs48/45. This research indicates the regions of both antigens that should, or should not, be included in next-generation vaccines for both Pfs230 and Pfs48/45. Other efforts with Pfs48/45 describe how stabilizing the structure can improve the induced immune response, leading to better outcomes in preclinical models.
The TBV approach is particularly important to malaria eradication efforts. When used in conjunction with other malaria control tools or vaccines targeting a different part of the parasite’s lifecycle, a vaccine able to protect the individual—and the mosquito—from parasite transmission could help push a geographic region past the threshold of control and accelerate elimination toward the ultimate goal of parasite eradication.
Finally, multistage malaria vaccines are an exciting next-generation approach, as they would target more than one stage of the Plasmodium parasite’s lifecycle to improve overall malaria vaccine efficacy. For example, combining anti-infection and blood-stage (anti-disease) vaccines may lead to synergistic benefits that enable two moderately efficacious approaches to exhibit profound effects.
Likewise, combining anti-infection vaccines and TBVs may also lead to a synergistic effect to target bottlenecks in the parasite lifecycle. The incorporation of a TBV into other vaccine approaches could provide an indirect benefit by also blocking transmission of the parasite within communities. Over time, the transmission-blocking component would decrease the levels of the Plasmodium falciparum parasite in the community, and consequently, the probability an individual would become infected.
PATH contributed to establishing guidance for preclinical and early clinical testing of multistage CSP-based and blood-stage vaccine approaches by helping to convene an expert consultation that resulted in several key recommendations to inform the development of multistage malaria vaccines.
Monoclonal antibodies to prevent malaria
Monoclonal antibodies (mAbs) are effective tools in fighting infectious diseases in young children. They also have the potential to offer high-level protection against infection and onward transmission of malaria.
In alignment with the WHO’s preferred product characteristics for mAbs for malaria prevention, PATH’s work covers both anti-infection and transmission-blocking mAbs targeting malaria. These mAbs are likely to have the greatest utility in highly seasonal settings by providing rapid, potent, short-term protection in vulnerable children. Anti-infection mAbs may also help with preventing malaria in pregnancy where current tools are insufficient.
Compared to vaccines, mAbs have the potential to be given as a single shot and have higher efficacy. In highly seasonal settings, where most of the malaria occurs over four to five months, a single mAb shot could be administered just before the malaria season and provide protection for the whole season.
In addition, mAbs are administered directly into the body versus “asking” the body to develop an antibody, as with a vaccine. That removes several variables related to how a particular person might respond to a vaccine, which can vary from person to person.
In collaboration with Ehime University, Eisai Co, Ltd., and GlaxoSmithKline, PATH is currently working on the preclinical development of an anti-infection mAb targeting the circumsporozoite protein on the P. falciparum parasite. This work is funded in part by the Global Health Innovative Technology Fund in Japan.
In addition, we partnered with Radboud University Medical Center in Nijmegen, the Netherlands, on a first-in-human clinical trial of a transmission-blocking mAb, TB31F. The study found that it was safe, well tolerated, and prevented transmission of the parasite to mosquitoes for three months using an ex vivo assay.
A critical next step is to determine whether TB31F blocks parasite transmission under natural transmission conditions (i.e., to mosquitoes feeding on the skin of infected volunteers). PATH is collaborating with partners on this ongoing study. If successful, these data are expected to significantly accelerate and de-risk the development of transmission-blocking vaccines and mAbs.
Looking to the future
There’s a lot of exciting research happening around the globe on novel malaria vaccine candidates and mAbs to prevent malaria infection and/or transmission. PATH is grateful to be involved in several of these efforts, and we’re eager to see where our ongoing research takes us.
While scientists continue to search for the “next best thing” to stop malaria, it’s critical that the currently available interventions continue to be made accessible and affordable to those who need them most. As lessons from malaria elimination efforts in specific countries and regions have shown, it will take more than just one type of intervention to truly put an end to malaria.