Hookworm, a blood-feeding parasitic worm (or helminth), infects roughly 406 to 480 million people globally each year. The infection results in serious health consequences, particularly for young children and pregnant people in low- and middle-income countries (LMICs). While treatments for hookworm are available and affordable, they’re often only partially effective, and increasing reinfection rates and emerging drug resistance are a growing concern.
Hookworm and malaria are the leading parasitic causes of anemia, a condition where there’s a lack of sufficient healthy red blood cells to carry oxygen around the body. While infections with these two parasites cause anemia in different ways, the health outcomes are the same and co-infections are common in many areas.
Texas Children’s Hospital Center for Vaccine Development at Baylor College of Medicine, together with strategic partners, is developing a hookworm vaccine candidate that is showing high levels of protective immunity in a proof-of-concept clinical trial using a controlled human infection model, but there’s uncertainty about LMIC interest in a standalone vaccine.
Baylor recently funded PATH to conduct a study to better understand stakeholder perspectives in four countries on potential use cases and target populations for a human hookworm vaccine and to explore the idea of a hookworm/malaria combination vaccine. The Democratic Republic of Congo (DRC), Ghana, Malawi, and Uganda were selected for the study because they all have demonstrated hookworm and malaria disease burdens.
Providing protection against both diseases with a single vaccine could offer an efficient, effective, and affordable way to improve health in LMICs. The stakeholders interviewed in our study indicated that a hookworm/malaria combination vaccine would be a highly valuable intervention in the fight against these parasites.
Insights from local stakeholders
Hookworm is one of the most important neglected tropical diseases in terms of its major impact on child and maternal health. Hookworm infections can lead to increased infant mortality, as well as longer-term health consequences such as impaired cognitive development in children.
Current interventions to prevent hookworm infection include mass drug administration (MDA) and individualized treatment with anti-parasitic drugs, as well as the use of proper footwear and improved water, sanitation, and hygiene. While these are effective in some settings, drug failures have been reported and don’t prevent hookworm reinfection. As a result, the burden of hookworm disease in highly endemic areas of Africa, Asia, and Latin America has not declined. A vaccine could reduce reinfection rates, provide longer-lasting protection, avoid drug resistance, and contribute to improving long-term health outcomes.
Several hookworm vaccine candidates have been studied, and Baylor’s selected candidate has now shown high levels of protective immunity in a recent clinical trial. However, many funders, vaccine manufacturers, and country-level decision-makers in endemic countries are unenthusiastic about supporting a standalone hookworm vaccine.
PATH conducted 16 in-depth stakeholder interviews in the DRC, Ghana, Malawi, and Uganda, four countries with confirmed hookworm and malaria burdens. The interviews sought their perspective on potential use cases and target populations for a hookworm vaccine and the concept of a hookworm/malaria combination vaccine. All stakeholders interviewed had experience in the prevention of disease caused by either or both parasites, as well as knowledge about vaccine introduction decision-making and implementation.
Country-level vaccination decision-makers are one of the most important stakeholders to involve when considering new vaccine approaches. They’re the ones ultimately deciding whether or not to introduce new vaccines into their country’s immunization schedule. As such, it’s critical to take their perspectives into account when designing candidate vaccines and considering potential new combinations.
A “highly valuable” combination vaccine
All four study countries have ongoing hookworm prevention and control strategies that include MDA, but the target population and frequency of this intervention vary. National-level stakeholders in this study were well aware of the appreciable disease burden caused by hookworm, particularly its health impacts. However, only 38 percent considered it a serious public health problem in their country.
Consistent with this, the majority of stakeholders perceived a hookworm standalone vaccine as only “fairly valuable,” with only 2 of the 16 stakeholders interviewed considering it “highly valuable.” In contrast, almost all the stakeholders (13 of 16 interviewed) perceived a hookworm/malaria combination vaccine as “highly valuable,” citing a range of financial and programmatic benefits, such as better value for money and expanded coverage opportunities through the existing malaria vaccination platform.
The stakeholders noted several factors that would increase the perceived value of a hookworm/malaria combination vaccine. For instance, vaccines with high efficacy and low incremental cost would be especially appealing. Another important factor is whether the combination vaccine could provide protection in fewer than the required four doses for the current standalone malaria vaccines.
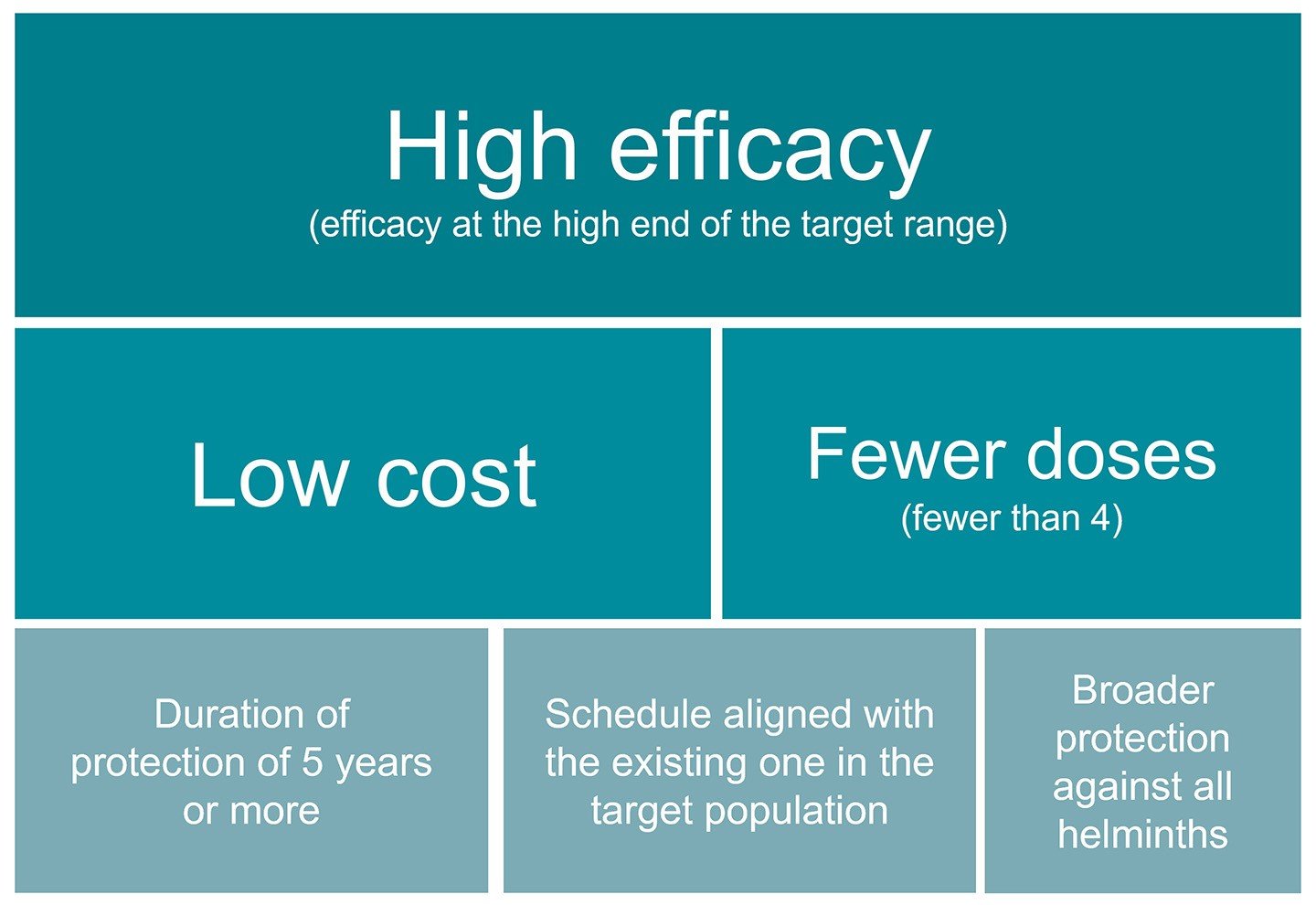
This figure highlights the factors that would increase the value of a hookworm/malaria combination vaccine as identified by stakeholders in the study. The size of the boxes represents the approximate number of stakeholders who mentioned each factor.
Combining a hookworm vaccine candidate with one of the malaria vaccines currently being implemented in sub-Saharan Africa could offer a promising and innovative pathway to addressing both major parasites at the same time. Other potential combinations to consider could include hookworm vaccine with vaccines for other enteric pathogens, such as Salmonella enterica, the causes of typhoid and paratyphoid fever. This approach could also serve as a model for a broader effort to integrate vaccines against other helminths into routine immunization schedules.
Childhood immunization programs have expanded drastically over the last 50 years with the development and introduction of multiple vaccines. This has resulted in a crowded and complex immunization landscape. Country immunization programs are challenged by the practicalities of accommodating additional new vaccines, including concerns about financing, cold chain capacity, and parent and health care provider acceptability of more injections for children.
New combination vaccines could help alleviate these challenges by offering the possibility of introducing vaccines against a broader range of pathogens—or in this case, two parasites—without increasing the number of separate vaccine administrations.
What’s next?
While this study had a relatively small sample size, the results are clear. A hookworm/malaria combination vaccine is likely to be perceived as highly valuable in countries with relevant epidemiology for both diseases.
As research continues on a standalone hookworm vaccine candidate, to prepare and de-risk advanced product and clinical development plans, funders and developers should consider the preferred vaccine characteristics identified by the stakeholders in this study. It’s also important for countries to continue collecting local disease burden data and raising awareness about hookworm prevention to build support for future vaccines.
In the fight against hookworm, these stakeholder perspectives are bringing attention to an innovative vaccine development approach that could help reduce parasite-induced illness in more ways than one.