2025 has been a big year on the road to respiratory syncytial virus (RSV) prevention. In March, the World Health Organization (WHO) prequalified a maternal RSV vaccine (Abrysvo®, Pfizer, Inc.), which it has recommended for global use. The Pan American Health Organization (PAHO) has also recommended the vaccine. In July, Gavi, the Vaccine Alliance committed to opening a funding window for an RSV maternal immunization program, marking a milestone on the path to introduction in low- and middle-income countries (LMICs).
With WHO prequalification of the single-dose vial of the maternal vaccine, now is the time for countries to begin exploring how they can introduce vaccines and other tools for RSV prevention. An important next step now is to accelerate the pathway to WHO prequalification for a multi-dose vial presentation that’s better suited for low- and middle-income markets, which could be as soon as 2026. (Long-acting monoclonal antibodies are also WHO-recommended for protecting infants from RSV, but price and supply are currently barriers to access for many LMICs and will require market shaping to overcome.)
Recommendations and guidance from WHO can provide a foundation for countries as they begin this work. While there is likely still a considerable runway before the maternal vaccine is introduced in LMICs, the stage is set to begin preparations that will benefit countless children down the line.
Vaccinating mothers, protecting newborns
RSV is the leading cause of severe respiratory infections and hospitalization in infants and young children, and the virus is so widespread that nearly all children contract it before 2 years of age. There are stark disparities in RSV mortality between low- and high-income settings: 98 percent of pediatric RSV deaths occur in low- and middle-income economies, and many children never make it to the hospital.
“RSV is a major cause of infant illness and death, especially in low-resource settings where some children never even reach care,” said Clint Pecenka, director of PATH’s RSV vaccine project. “Maternal vaccination offers a vital chance to reduce this burden—protecting babies early and easing the pressure on families and health systems.”
In this context, preventing severe RSV disease before it starts is all the more critical. The maternal RSV vaccine helps protect babies when they are the most vulnerable: given in pregnancy, the vaccine enhances a pregnant woman’s immunity and increases natural antibody transfer to the baby in their first months of life. WHO globally recommends vaccination in the third trimester of pregnancy, as defined by local guidelines.
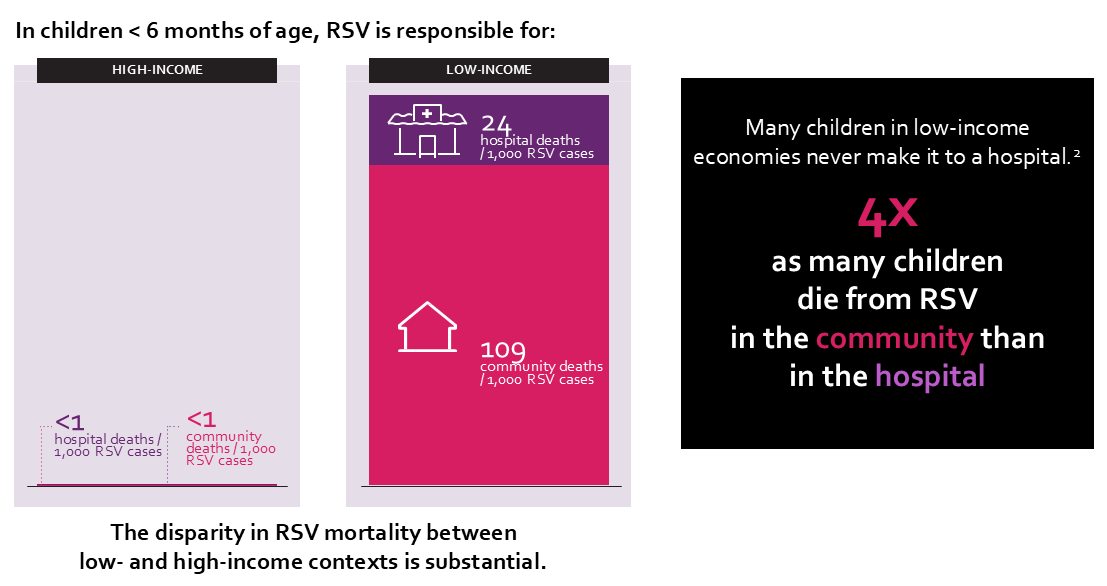
RSV disease burden in low- and high-income contexts. Graphic: WHO; PATH; RRD.
What RSV vaccines could mean for children
Pfizer’s maternal vaccine has shown impressive results in clinical trials. A Phase 3 clinical study found that protection from the vaccine remains high through 6 months after birth, with 82 percent efficacy in the first 90 days and 70 percent after 180 days. These data tell us that the vaccine can help protect children during their first 6 months of life, when RSV poses the greatest threat to their health.
And real-world data from countries that have introduced the maternal RSV vaccine, like Argentina, are emerging and showing high vaccine effectiveness, especially against severe disease.
Further, RSV imposes a substantial strain on health systems and households, causing millions of hospitalizations and placing a burden on parents and caregivers. Introducing maternal vaccines could not only keep children out of the hospital, but could also avert health care costs and free up resources for other health priorities.
“Our cost-of-delivery analyses in Kenya, Ghana, and Mozambique show that estimated costs of delivering new maternal vaccines such as for RSV are within the range of other routine childhood vaccine costs,” said Ranju Baral, a senior health economist at PATH. “Modeling studies also indicate that these vaccines can be cost-effective in many LMICs, which will have an opportunity to be confirmed once vaccine pricing for LMIC markets is available. In short, there’s reason to be optimistic that maternal RSV vaccines could be a smart investment in maternal and child health where needed most.”
Expanding access to maternal vaccines
WHO’s recent recommendations on RSV vaccines build on a strong track record for maternal immunization. Maternal vaccines for diseases like tetanus, influenza, and pertussis have been making a difference for years, and new ones are in development for pathogens such as Group B Streptococcus. Maternal vaccines boast some distinct advantages—not only do they provide protection to two people at once, but they can also help infants when they’re too young to receive certain vaccines.
As more maternal vaccines become available, PATH is conducting research in Africa to gain a fuller picture of adoption readiness, exploring delivery requirements in Ghana, Senegal, Tanzania, and Zambia. PATH's research in these countries—as well as Mozambique, Vietnam, and five countries in the PAHO region—is also painting a fuller picture of how well current antenatal care visit timing aligns with recommended RSV maternal vaccination windows to inform implementation decision-making.
“Understanding how antenatal care and immunization programs intersect will be critical,” said Sadaf Khan, a program advisor for maternal, newborn, and child health at PATH. “We’re working to identify potential programmatic challenges, workforce readiness, and other gaps so that countries can equip themselves to roll out these vaccines.”
Turning readiness into reality
With the single-dose vial presentation of maternal RSV vaccines now prequalified, this is a key moment to prioritize planning for their rollout, especially given that RSV is a new disease target for many countries. As countries begin planning for RSV prevention, they’ll need to weigh the local impact of the disease, the cost and benefits of available products, and how well those products fit within their health systems. By expanding RSV surveillance efforts, countries can make the case for introducing vaccines and better understand where they can make the greatest impact.
Other key steps will include preparing delivery platforms via antenatal care and immunization programs, raising awareness among healthcare providers and caregivers, and ensuring systems are ready to monitor safety and effectiveness. Long-term success will also depend on workforce readiness and securing funding.
WHO, PATH, and other contributors have developed a suite of communications materials that cover RSV disease, new prevention tools, and delivery considerations. These tools can help support informed decision-making and implementation planning around RSV and relevant interventions.
Maternal RSV vaccines have exciting potential to protect infants from a deadly threat—one that has for too long been overlooked. By setting the stage for introduction, we can ensure that countries are well equipped to implement this lifesaving intervention in the coming years.