They are tired, and they want a break. That’s how community health workers in Senegal describe the women who visit their village health huts for family planning—women who often have had one baby after another.
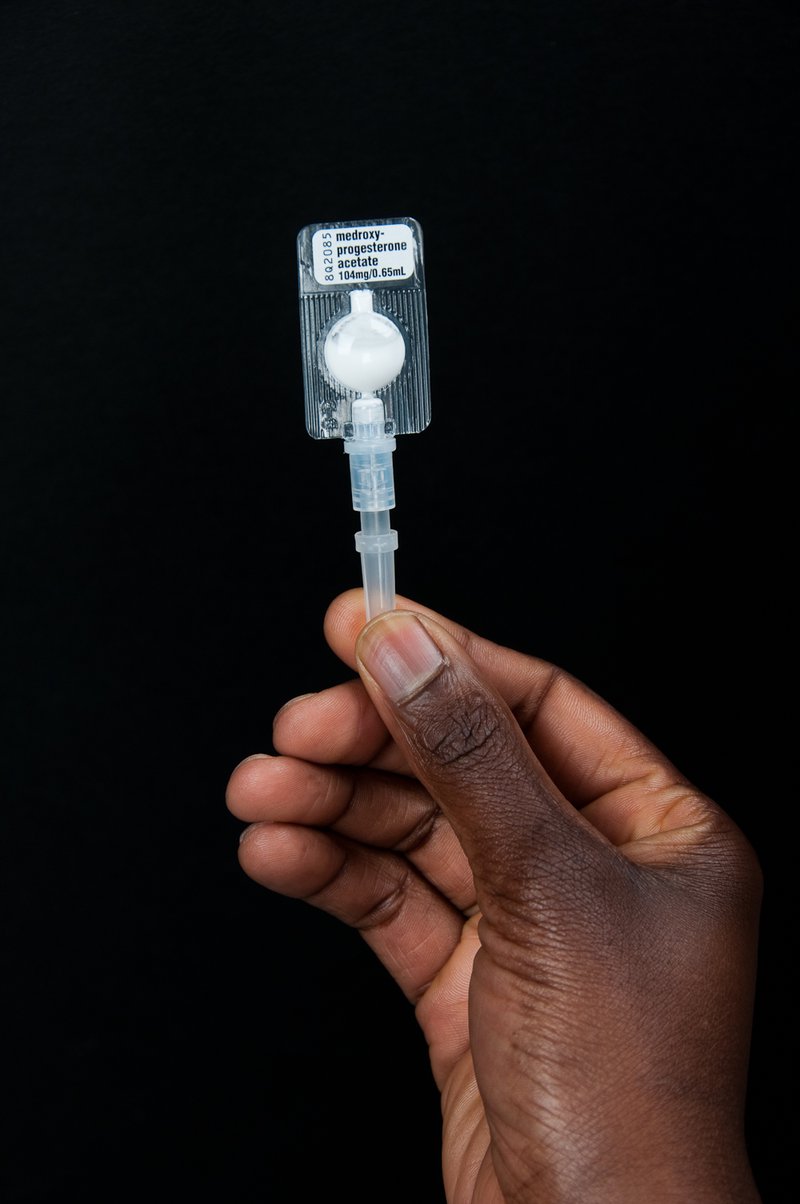
A new form of injectable contraception is small, light, and very easy to use. Photo: PATH/Patrick McKern.
“Some of the women are educated and some are not, but they are smart,” said a health worker, one of several interviewed as part of a study of a new choice in contraception. “They worry about the health consequences of multiple pregnancies.”
About a third of maternal deaths could be avoided by delaying motherhood, spacing births, preventing unintended pregnancy, and avoiding unsafely performed abortions. But that requires family planning services and methods that meet the reality of women’s lives. In Senegal, the most popular method—injectable contraceptives—is not available in village health huts. That’s about to change as a result of PATH’s innovative approach.
Rethinking injectable contraceptives
Injectable contraceptives like Depo-Provera® have some big advantages: they’re safe, there is almost no risk of an unintended pregnancy, and one shot provides protection for three months. And, they’re discreet. “This is a small village,” said a second Senegalese health worker. “Everyone knows everyone, and everyone knows what everyone else is doing.”
Village health huts are staffed by birth attendants and community volunteers, not the more highly trained health workers who typically give the injections at health clinics in larger towns. That means many women in remote areas have to travel long distances to get injectable contraceptives.
The introduction of a potentially groundbreaking new form of injectable contraceptive, called Sayana® Press, promises to change that. Sayana Press is prepackaged in the PATH-developed Uniject™ autodisable injection system—a small bubble of plastic prefilled with a single dose and attached to a short needle. The device is small, light, and very easy to use. In other words, it is uniquely suited for community health workers.
Getting contraceptives to the village
With the help of many funders and partners, PATH is getting ready to bring up to 12 million doses of Sayana Press to women in Senegal, Burkina Faso, Niger, Uganda, and Bangladesh over the next three years. The effort will be highlighted at the International Conference on Family Planning in Addis Ababa, Ethiopia, next week.

Cathy Ndyiaye, who recently finished her PhD in Public Health at the University of Montreal, came home to Senegal to lead the project. Photo: PATH/Siri Wood.
“This product brings about a major innovation in service delivery by allowing, for the first time, the availability of an injectable contraceptive in health huts at the community level,” says Cathy Ndiaye, PATH’s Senegal coordinator for the product introduction. “With Sayana Press, we know we will reach women in villages who have been left at the margin of contraceptive distribution for way too long.”
Delivering a better chance at health
Cathy is working closely with the Senegalese Ministry of Health and partners on a challenging task—almost a million units of Sayana Press are expected to be procured and shipped from Europe for use in Senegal’s four pilot introduction regions, and 3,700 health providers need to be trained.
The work is central to achieving one of PATH’s key goals—improving the health of women and their children. When contraceptives meet women’s needs and make it into villages and community health huts, women have more control over the timing and spacing of their children and a better chance at a healthy life.
Sayana Press and Depo-Provera are registered trademarks of Pfizer, Inc. Uniject is a trademark of BD.
