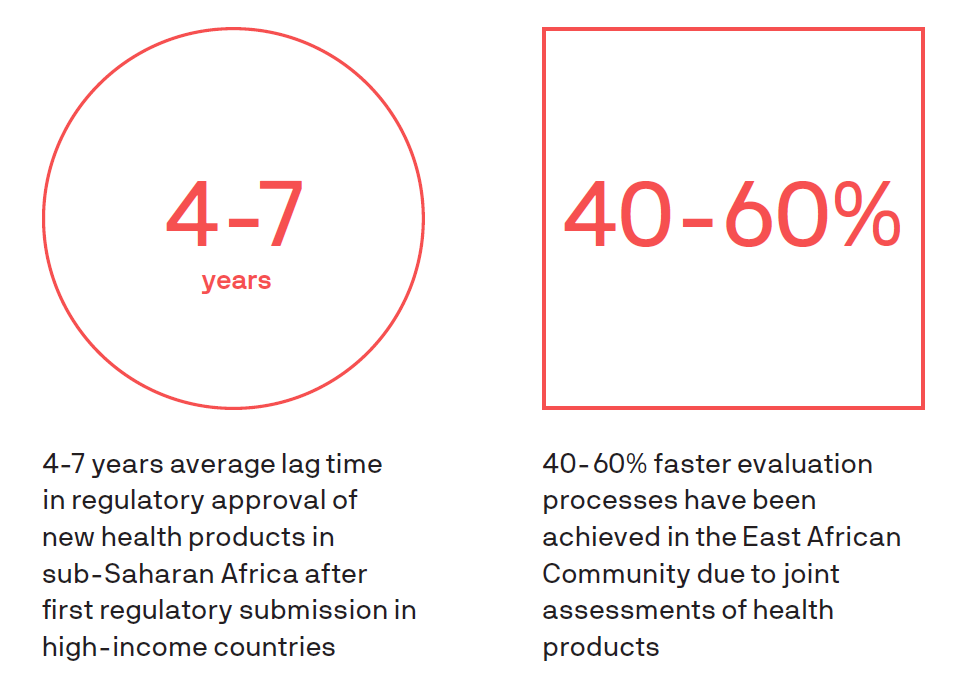
As African countries advance efforts to achieve universal health coverage, there is widespread recognition that we will not reach our goals without innovation of all kinds. In particular, the need for R&D to advance new tools—including vaccines, drugs, diagnostics, devices, data, and digital tools—to deliver high-quality, affordable health care has been elevated as a priority. Regulatory approvals are essential in ensuring the quality, safety, and efficacy of health products, but when regulatory processes are not aligned across African countries, these tools are prevented or delayed from reaching those who need them most. In addition, many regulatory agencies are under-resourced and over-burdened, creating bottlenecks and backlogs.
Efforts are underway to harmonize regulatory processes across Africa, but many are nascent and yet to realize their potential. While it may not seem inherently connected, regulatory harmonization will play an important role in African countries’ abilities to achieve their universal health coverage goals.
In a recent analysis, PATH demonstrated the potential impact of regulatory harmonization in real terms—lives saved. The analysis modeled how many lives could be saved as a result of accelerated access to new or improved drugs aimed at treating postpartum hemorrhage and pneumonia, two leading causes of death among women and children in Africa. The study found that harmonization of regulatory approvals for these two medicines alone could contribute to more than 23,000 lives saved in eastern and southern Africa.
This topic was front and center at the recent Africa Health Agenda International Conference, held in Kigali, Rwanda, from March 5 through 7, 2019. At the conference, PATH hosted a session titled “Patients Are Waiting: Accelerating Access Through Strengthening Regulatory Harmonization of Medical Products and Health Innovations in Africa,” which looked specifically at the role of regulatory harmonization in accelerating innovation to achieve health for all in Africa.

Participants during PATH’s facilitated session on accelerating access through strengthening of regulatory harmonization at the Africa Health Agenda International Conference. Photo: PATH/Douglas Waudo.
As Anthony Okoth, country director for PATH in Kenya, explained at the session, “An important part of achieving health for all is focusing on advancing needed new health innovations, which include products and system innovations, to ensure they are scaled up and access is achieved for all populations.”
The session delved into how innovation can be effectively used to advance universal health coverage and focused on one challenge in particular: the fact that complex and disparate regulatory processes across the African continent too often delay health products from reaching those that need them most and prevent those products from having the impact they could.
Building collective support for research
PATH is committed to building broad-based civil-society support for R&D. To that end, PATH has supported the establishment of two strong coalitions that advocate for R&D in Africa: the Coalition for Health Research and Development (CHReaD) in Kenya and the South African Health Technologies Advocacy Coalition (SAHTAC) in South Africa. These groups engage with researchers, policymakers, and other players in the health sector to push for development of enabling policies and increased investments in R&D. In Kigali, CHReaD and SAHTAC representatives shared their experiences on how the coalitions are catalyzing action on health R&D through coordinated advocacy efforts to increase access to lifesaving products, technologies, and innovations.

New ideas for lifesaving health products are generated every day, but they are only impactful if they reach the people who need them most. Photo: PATH/Eric Becker.
Yolanda Moyo, representing SAHTAC, said, “To accelerate regulatory harmonization efforts and improve access to essential health products for underserved populations, political commitment must be paired with assurance of resources. Sustainable funding must be mobilized from donor and domestic funders, harmonization efforts must be expanded to cover different types of products and across regulation phases, and the AU Model Law should be adopted and converged into law by each member state.”
Dr. Mary Waiyego, a neonatologist at Pumwani Hospital in Nairobi, offered insights as a frontline health care worker on how innovation in health systems improves access to health services as well as quality of care, averting deaths and illnesses among neonates and children, enabling them to grow healthy beyond their fifth birthday. “Research and development is critical to ensuring that high-impact, affordable health technologies reach the people who need them most,” Dr. Waiyego stated.
“Research and development is critical to ensuring that high-impact, affordable health technologies reach the people who need them most.”— Dr. Mary Waiyego, neonatologist at Pumwani Hospital in Nairobi
PATH believes that health innovation must be included in the universal health coverage agenda. In particular, there is a need for countries to:
- Strengthen and streamline regulatory systems to support research and to get products approved efficiently while also ensuring quality.
- Ensure adequate investments for R&D for appropriate health products and technologies to meet the health needs of Africans.
- Embrace innovation and put systems in place to support scale-up to increase public health impact across the continent.
- Nurture a culture of accountability around commitments to universal health coverage, including innovation.
PATH advocates for increased investment in health R&D, evidence-informed policy change and implementation, and a streamlined regulatory system as effective and efficient ways of achieving universal health coverage. Through partnership, evidence generation, and accountability measures, PATH is committed to elevate this common message to ensure that health R&D is prioritized as a means of achieving the goal of health for all.
For more information: Making the Case: How Regulatory Harmonisation Can Save Lives in Africa



